
Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Oxidation Reaction: It is a chemical reaction in which gain of oxygen or loss of hydrogen takes place.
For example:
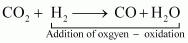
In equation (i), H2is oxidized to H2O and in equation (ii), Cu is oxidised to CuO.
Reduction Reaction: It is a chemical reaction in which loss of oxygen or gain of hydrogen takes place.
For example:
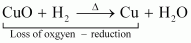
In equation (i), CO2is reduced to CO and in equation (ii), CuO is reduced to Cu.