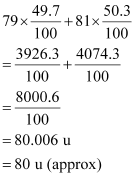If bromine atom is available in the form of, say, two isotopes 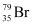 (49.7%) and
(49.7%) and 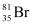 (50.3%), calculate the average atomic mass of bromine atom.
(50.3%), calculate the average atomic mass of bromine atom.
It is given that two isotopes of bromine are
![]() (49.7%) and
(49.7%) and
![]() (50.3%). Then, the average
atomic mass of bromine atom is given by:
(50.3%). Then, the average
atomic mass of bromine atom is given by: