
The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 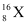 and
and 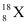 in the sample?
in the sample?
It is given that the average atomic mass of the sample of element X is 16.2 u.
Let the percentage of isotope 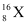 be y %. Thus, the percentage of isotope
be y %. Thus, the percentage of isotope 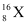 will be (100 - y) %.
will be (100 - y) %.
Therefore,
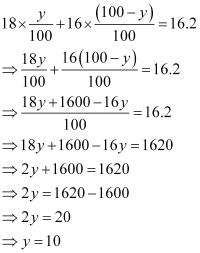
Therefore, the percentage of isotopeis 10%.
And, the percentage of isotopeis (100 - 10) % = 90%.