MCQ Solution: Solid State
CsBr has b.c.c. structure with edge length 4.3 A. The shortest inter ionic distance in between Cs+ and Br– is:
1) 4.3
2) 1.86
3) 7.44.
4) 3.72
SOLUTION
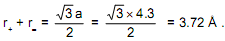
CsBr has b.c.c. structure with edge length 4.3 A. The shortest inter ionic distance in between Cs+ and Br– is:
1) 4.3
2) 1.86
3) 7.44.
4) 3.72
SOLUTION