Quest
Ionic Equilibrium
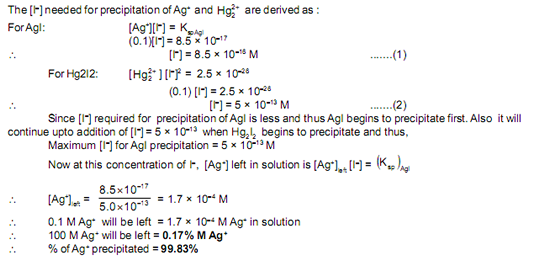
A solution contains a mixture of Ag+ (0.10 M) and Hg22+ (0.10 M) which are to be separated by selective precipitation. Calculate the maximum concentration of iodide ion at which one of them gets precipitated almost completely. What % of that metal ion is precipitated? Ksp of AgI = 8.5 × 10–17and Ksp of Hg2I2 = 2.5 × 10–26 1) 0.17%
2) 9.983%
3) 83%.
4) 99.83%
SOLUTION