NCERT Solution: Chemical Reactions and Equations
‘X’ is copper (Cu) and the black-coloured compound formed is copper oxide (CuO). The equation of the reaction involved on heating copper is given below.
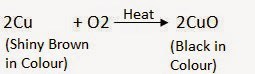
Iron articles are painted because it prevents them from rusting. When painted, the contact of iron articles from moisture and air is cut off. Hence, rusting is prevented.
Oil and fat containing food items flushed with nitrogen because nitrogen acts as an antioxidant and it prevent them from being oxidised.
Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Answer
Corrosion is defined as a process where materials, usually metals, deteriorate as a result of a chemical reaction with air, moisture, chemicals, etc.
For example, iron, in the presence of moisture, reacts with oxygen to form hydrated iron oxide.
4Fe + 3O2 + nH2O → 2Fe2O3.nH2O
Rancidity is the process of oxidation of fats and oils that can be easily noticed by the change in taste and smell is known as rancidity.
For example, the taste and smell of butter changes when kept for long.
(a) Boiling of water to give water vapour
(b) Melting of ice to give water
(c) Dissolution of salt in water
(d) Combustion of Liquefied Petroleum Gas (LPG)
Ans. (d) Combustion of Liquefied Petroleum Gas (LPG)
Explanation: Combustion of any substance is a chemical change because new substance is formed after combustion.
2. The following reaction is an example of a
4NH3(g) + 5O2 → 4NO + 6H2O (g)
(i) displacement reaction
(ii) combination reaction
(iii) redox reaction
(iv) neutralisation reaction
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (iii) and (iv
Ans. (c) (i) and (iii)
Explanation: In this reaction, oxygen is displacing hydrogen from ammonia; hence it is a displacement reaction. Moreover, nitrogen is getting oxidized and oxygen is getting reduced. Hence, this is redox reaction.
3. Which of the following statements about the given reaction are correct?
3Fe(s) + 4H2O(g) →Fe3O4(s)+ 4H2(g)
(i) Iron metal is getting oxidized
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidizing agent
(a) (i), (ii) and (iii)
(b) (iii) and (iv)
(c) (i), (ii) and (iv)
(d) (ii) and (iv)
Ans. (c) (i), (ii) and (iv)
Explanation: Oxygen is being added to iron, hence iron is getting oxidized. Oxygen is removed from water; hence water is getting reduced. Water is providing oxygen; hence water is the oxidizing agent.
4. Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Ans. (a) (i) and (ii)
Explanation: Evaporation can happen only after absorbing heat. Sublimation of a solid into gas can happen only after absorbing heat. Hence, (iii) and (iv) are endothermic processes. Rest are exothermic processes.
5. Three beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of
the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statement(s) is(are)correct?
(i) In beakers A and B, exothermic process has occurred.
(ii) In beakers A and B, endothermic process has occurred.
(iii) In beaker C exothermic process has occurred.
(iv) In beaker C endothermic process has occurred.
(a) (i) only
(b) (ii) only
(c) (i) and (iv)
(d) (ii) and (iii)
Ans. (c) (i) and (iv)
Explanation: Exothermic process will increase the temperature of beaker, while endothermic process will reduce the temperature of beaker.
6. A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the
observation?
(a) KMnO4 is an oxidising agent, it oxidises FeSO4
(b) FeSO4 acts as an oxidising agent and oxidises KMnO4
(c) The colour disappears due to dilution; no reaction is involved
(d) KMnO4 is an unstable compound and decomposes in presence of FeSO4 to a colourless compound.
Ans. (a) KMnO4 is an oxidising agent, it oxidises FeSO4 Explanation: Potassium permanganate is a potential oxidizing agent.
The purple colour was because of KMnO4 ; which disappears once all the permanganate in the solution is utilized
7. Which among the following is(are) double displacement reaction(s)?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2→CO2
(iv) CH4 +2O2→ CO2+H2O
(a) (i) and (iv)
(b) (ii) only
(c) (i) and (ii)
(d) (iii) and (iv)
Ans. (b) (ii) only
Explanation: In this reaction, sodium and barium are displacing each other from their respective salts. Hence, it is a double displacement reaction.
Which among the following statement(s) is(are) true? Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride
(ii) sublimation of silver chloride
(iii) decomposition of chlorine gas from silver chloride
(iv) oxidation of silver chloride
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iv) only
Ans. (a) (i) only
Explanation: When silver chloride is kept in sunlight: it turns to grey because of formation of silver. This is a decomposition reaction.
Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanies by liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among
the following is (are) true about slaking of lime and the solution formed?
(i) It is an endothermic reaction
(ii) It is an exothermic reaction
(iii) The pH of the resulting solution will be more than seven
(iv) The pH of the resulting solution will be less than seven
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Ans. (b) (ii) and (iii)
Explanation: Slaking of lime is an exothermic reaction which is evident from liberation of heat. Oxides and hydroxides of metals are alkaline. Hence, pH of the resulting solution will be more than 7.
10. Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
(a) (i) only
(b) (ii) only
(c) (iv) only
(d) (ii) and (iv)
Ans. Explanation: (c) (iv) only
Refer to explanation to question 7.
11. Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is
(a) 1:1
(b) 2:1
(c) 4:1
(d) 1:2
Ans. (b) 2:1
Explanation: In 1 mole water molecule, there are 2 mole hydrogen and 1 mole oxygen.
12. Which of the following is(are) an endothermic process(es)?
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water
(a) (i) and (iii)
(b) (ii) only
(c) (iii) only
(d) (ii) and (iv)
Ans. (d) (ii) and (iv)
Explanation: When a solid change into gas or a liquid change into gas, it happens because of absorption of heat. Hence, these are endothermic processes.
13. In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be
used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Ans. (b) Lead acetate
Explanation: We need a source of lead for making lead iodide, hence options (c) and (d) are ruled out. Lead sulphate is insoluble, so it cannot be used.
14. Which of the following gases can be used for storage of fresh sample of an oil for a long time?
(a) Carbon dioxide or oxygen
(b) Nitrogen or oxygen
(c) Carbon dioxide or helium
(d) Helium or nitrogen
Ans. (d) Helium or nitrogen
Explanation: Oxygen is an oxidizing agent and hence it cannot be used. Helium is an inert gas and hence it can be used. Nitrogen is among the least reactive gases and it is much cheaper than helium. Hence, in most of the cases, Nitrogen is used in packets of oily food to prevent rancidity.
15. The following reaction is used for the preparation of oxygen gas in the laboratory
2KCIO3(s) heat, catalyst-> 2KCI s +3O2 (g)
Which of the following statement(s) is(are) correct about the reaction?
(a) It is a decomposition reaction and endothermic in nature
(b) It is a combination reaction
(c) It is a decomposition reaction and accompanied by release of heat
(d) It is a photochemical decomposition reaction and exothermic in nature
Ans. (a) It is a decomposition reaction and endothermic in nature.
Explanation: Potassium chlorate decomposes to give Potassium chloride and oxygen;
hence it is a decomposition reaction. Heat is being supplied to this reaction, so it is an endothermic reaction.
16. Which one of the following processes involve chemical reactions?
(a) Storing of oxygen gas under pressure in a gas cylinder
(b) Liquefaction of air
(c) Keeping petrol in a china dish in the open
(d) Heating copper wire in presence of air at high temperature
Ans. (d) Heating copper wire in presence of air at high temperature.
Explanation: When copper is heated at high temperature in presence of air, it undergoes oxidation to form copper oxide.
2Cu + O2 -> 2CuO
17. In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products
involved at reaction temperature?
(a) 2H2(l) + O2(l) -> 2H2O (g)
(b)2H2(g) + O2 (l)-> 2H2O (l)
(c) 2H2(g) + O2 (g)-> 2H2O (l)
(d) 2H2(g) + O2 (g)-> 2H2O (g)
ans C
Explanation: At room temperature, hydrogen and oxygen are available as gas, while water is available as liquid.
18. Which of the following are combination reactions?
(a) (i) and (iii)
(b) (iii) and (iv)
(c) (ii) and (iv)
(d) (ii) and (iii)
Ans. (d) (ii) and (iii)
Explanation: In these reactions, two reactants react to form a single product.
N2 g + 3H2 -> 2NH3
This is a combination reaction.
NaOH + CH3COOH → CH3COONa + H2O
This is neutralization reaction.
Ans. C2H5OH + CH3COOH → CH3COOC2H5 + H2O
This is neutralization reaction.