NCERT Solution: Metals and Non-metals
Hydrogen gas is evolved when dilute hydrochloric acid is added to a reactive metal.
When iron reacts with dilute H2SO4, iron (II) sulphate with the evolution of hydrogen gas is formed.
Fe (s) + H2SO4 (aq) → FeSO4(aq) + H2 (g)
When zinc is added to iron (II) sulphate then it will displace the iron from iron sulphate solution as shown in the following chemical reaction.
Zn (s) + FeSO4 (aq) → ZnSO4 (aq) + Fe (s)
(ii)

(iii) The ions present in Na2O are Na+ and O2- ions and in MgO are Mg2+ and O2- ions.

Ionic compounds have strong electrostatic forces of attraction between the ions. Therefore, it requires a lot of energy to overcome these forces. That is why ionic compounds have high melting points.
(i) Mineral: The naturally occurring compounds of elements are known as Mineral.
(ii) Ore: Minerals from which metals can be extracted profitably are known as ores.
(iii) Gangue: The impurities present in the ore such as sand, rocks etc are non as gangue.
The metals at the bottom of the reactivity series are mostly found in free state. For example: gold, silver, and platinum.
The chemical process used for obtaining a metal from its oxide is reduction. In this process, metal oxides are reduced by using suitable reducing agents such as carbon or by highly reactive metals to displace the metals from their oxides.
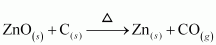
For example, zinc oxide is reduced to metallic zinc by heating with carbon.
Manganese dioxide is reduced to manganese by treatingit with aluminium powder. In this case, aluminium displaces manganese from its oxide.
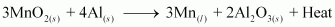
Oxides of more reactive metals are reduced by electrolysis.
Metallic oxides of zinc, magnesium and copper were heated with the following metals.
| Metal | Zinc | Magnesium | Copper |
| Zinc oxide | - | - | - |
| Magnesium oxide | - | - | - |
| Copper oxide | - | - | - |
In which cases will you find displacement reactions taking place?
Answer:
| Metal | Zinc | Magnesium | Copper |
| Zinc oxide | No reaction | Displacement | No reaction |
| Magnesium oxide | No reaction | No reaction | No reaction |
| Copper oxide | Displacement | Displacement | No reaction |