NCERT Solution: Structure of the Atom
It is given that two isotopes of bromine are
![]() (49.7%) and
(49.7%) and
![]() (50.3%). Then, the average
atomic mass of bromine atom is given by:
(50.3%). Then, the average
atomic mass of bromine atom is given by:
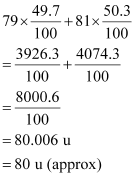
It is given that the average atomic mass of the sample of element X is 16.2 u.
Let the percentage of isotope 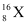 be y %. Thus, the percentage of isotope
be y %. Thus, the percentage of isotope 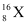 will be (100 - y) %.
will be (100 - y) %.
Therefore,
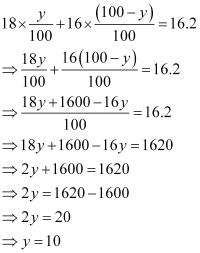
Therefore, the percentage of isotopeis 10%.
And, the percentage of isotopeis (100 - 10) % = 90%.
By Z = 3, we mean that the atomic number of the element is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron).
Therefore, the element with Z = 3 is lithium.
Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Answer
Mass number of X = Number of protons + Number of neutrons
= 6 + 6
= 12
Mass number of Y = Number of protons + Number of neutrons
= 6 + 8
= 14
These two atomic species X and Y have the same atomic number, but different mass numbers. Hence, they are isotopes.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
► False
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
► False
(c) The mass of an electron is about 1 / 2000times that of proton.
► True
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
► False
Rutherford's alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus
(b) Electron
(c) Proton
(d) Neutron
ANS (a) Atomic nucleus
Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers
ANS (c) different number of neutrons
Number of valence electrons in Cl -ion are:
(a) 16
(b) 8
(c) 17
(d) 18
ANS (b) 8
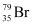 (49.7%) and
(49.7%) and 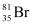 (50.3%), calculate the average atomic mass of bromine atom.
(50.3%), calculate the average atomic mass of bromine atom.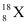 in the sample?
in the sample?